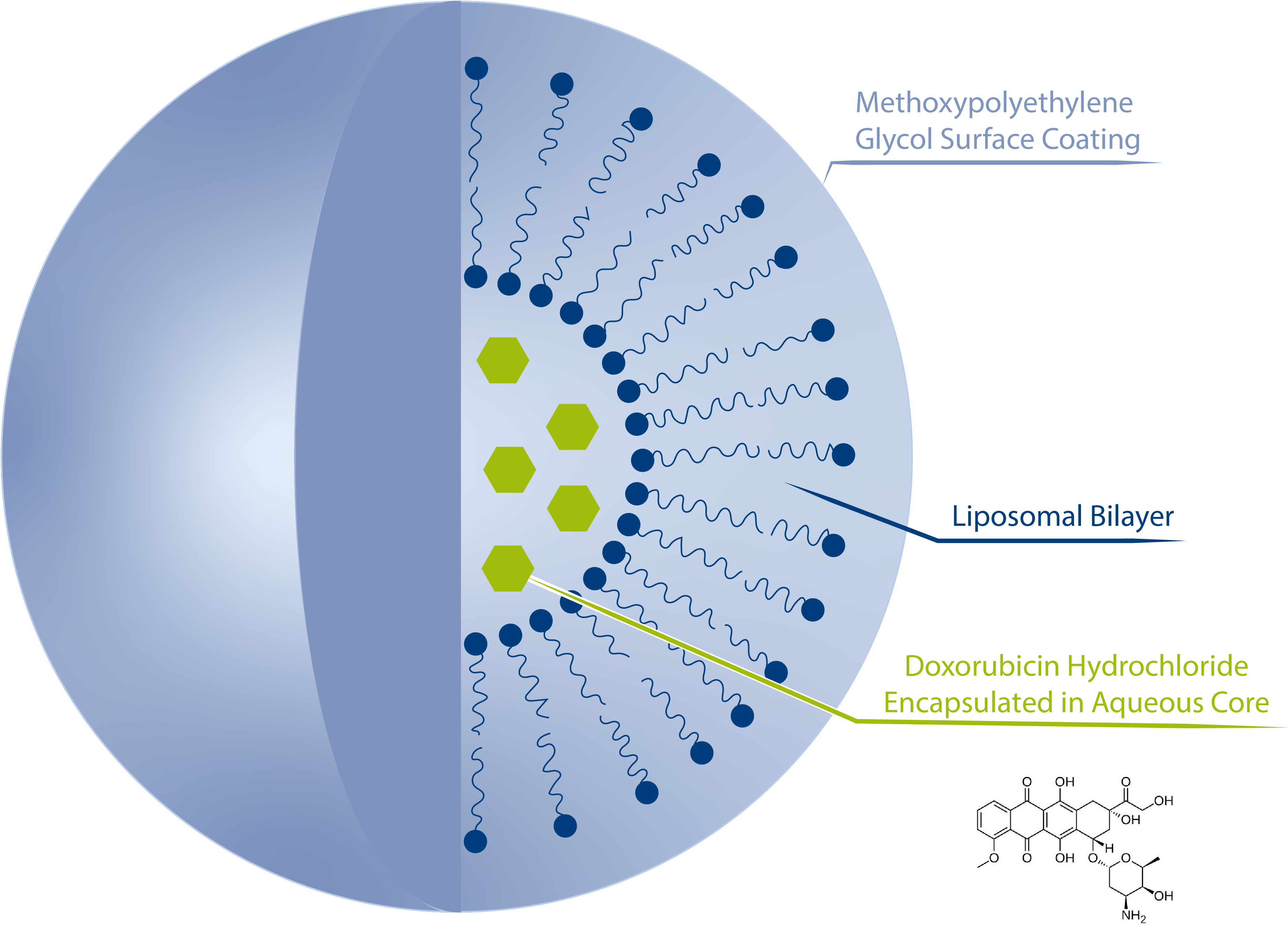
Introduction
Doxorubicin is a well-established chemotherapeutic agent frequently used in cancer treatment. Already in 1995, Doxil®, the brand name of the first liposomal Doxorubicin formulation, became the very first FDA-approved nano-drug. Encapsulation of Doxorubicin is a useful way to mitigate its cardiotoxicity and to ensure a high, stable dose as well as a prolonged circulation time of the drug in the human body [1]. Today, several liposomal Doxorubicin (Figure 1) formulations are available for clinical application and the accurate characterization of their physico-chemical properties - including size distribution, shape and physico-chemical stability - is a prerequisite for market approval. Asymmetrical Flow Field-Flow Fractionation (AF4) coupled with multiple detectors, providing particle size separation and characterisation in one system, has proven to be a powerful analytical setup to assess these critical quality attributes including from a regulatory [2] and a standardization [3] perspective. Besides the size distribution, precise knowledge of the surface charge (Zeta potential) is a key parameter towards tailor-made liposomal drug formulations with enhanced physico-chemical stability especially under physiological conditions. Here we present the application of Electrical Asymmetrical Flow Field-Flow Fractionation coupled with Multi Angle Light Scattering to derive both essential physico-chemical parameters, size distribution and surface charge, of a commercial liposomal Doxorubicin formulation.
Electrical Asymmetrical Flow Field-Flow Fractionation
Electrical Asymmetrical Flow Field-Flow Fractionation (EAF4) combines the principle of Electrical and Asymmetrical Flow Field-Flow Fractionation in one single channel with an electrical and a cross flow field applied simultaneously across the channel (Figure 2). By this means, EAF4 enables access to both size distribution and surface charge of a respective sample.

In order to obtain the surface charge from an EAF4 experiment, measurements with and without application of an electrical field have to be performed. The instrument software can then automatically calculate the Zeta potential via the following steps: Firstly, the ratio of the obtained retention times, with and without electrical field, allows the calculation of the drift velocity induced by field. Then, the effective electrical field is determined by taking into account the applied electrical current and the simultaneously measured conductivity of the eluent. A simple linear fit of drift velocity versus applied field yields the electrophoretic mobility, from which the surface Zeta potential can be derived.
Size Distribution and Surface Zeta Potential of Liposomal Doxorubicin
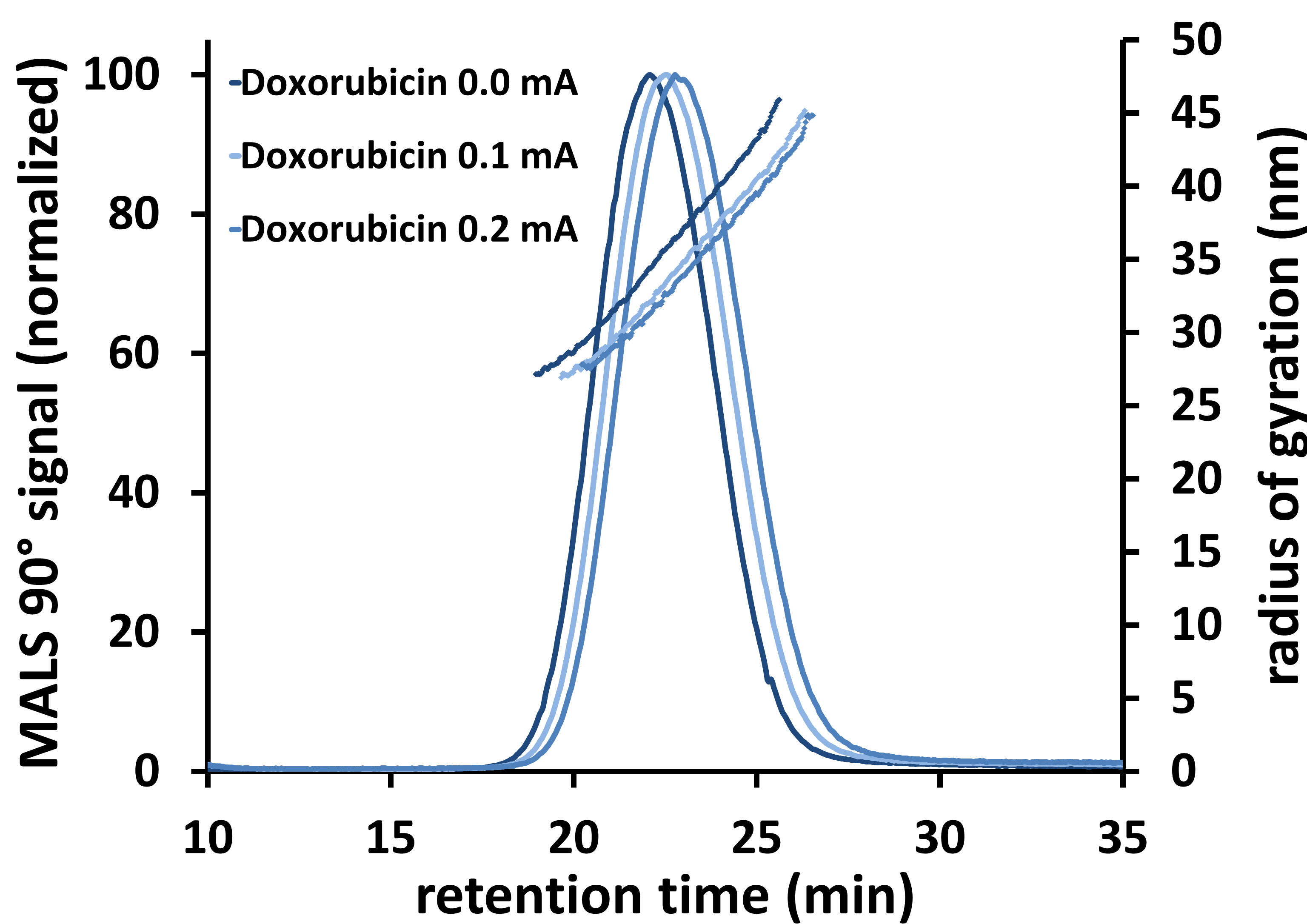
EAF4 measurements were performed using 0.5 mM NaCl as eluent at three different applied electrical currents (0.0 mA, 0.1 mA, 0.2 mA). In the experiments where a charge was applied, the top plate of the EAF4 channel was negatively charged. In addition, an online Multi Angle Light Scattering (PN3621 MALS) detector was used to derive the size distribution of the Liposomal Doxorubicin sample. Figure 3 displays the obtained EAF4-MALS fractogram indicating a broad size distribution of the investigated sample (radius of gyration Rg = 27-45 nm, Berry model fit) with no detrimental influence of the applied electrical field on the size distribution.
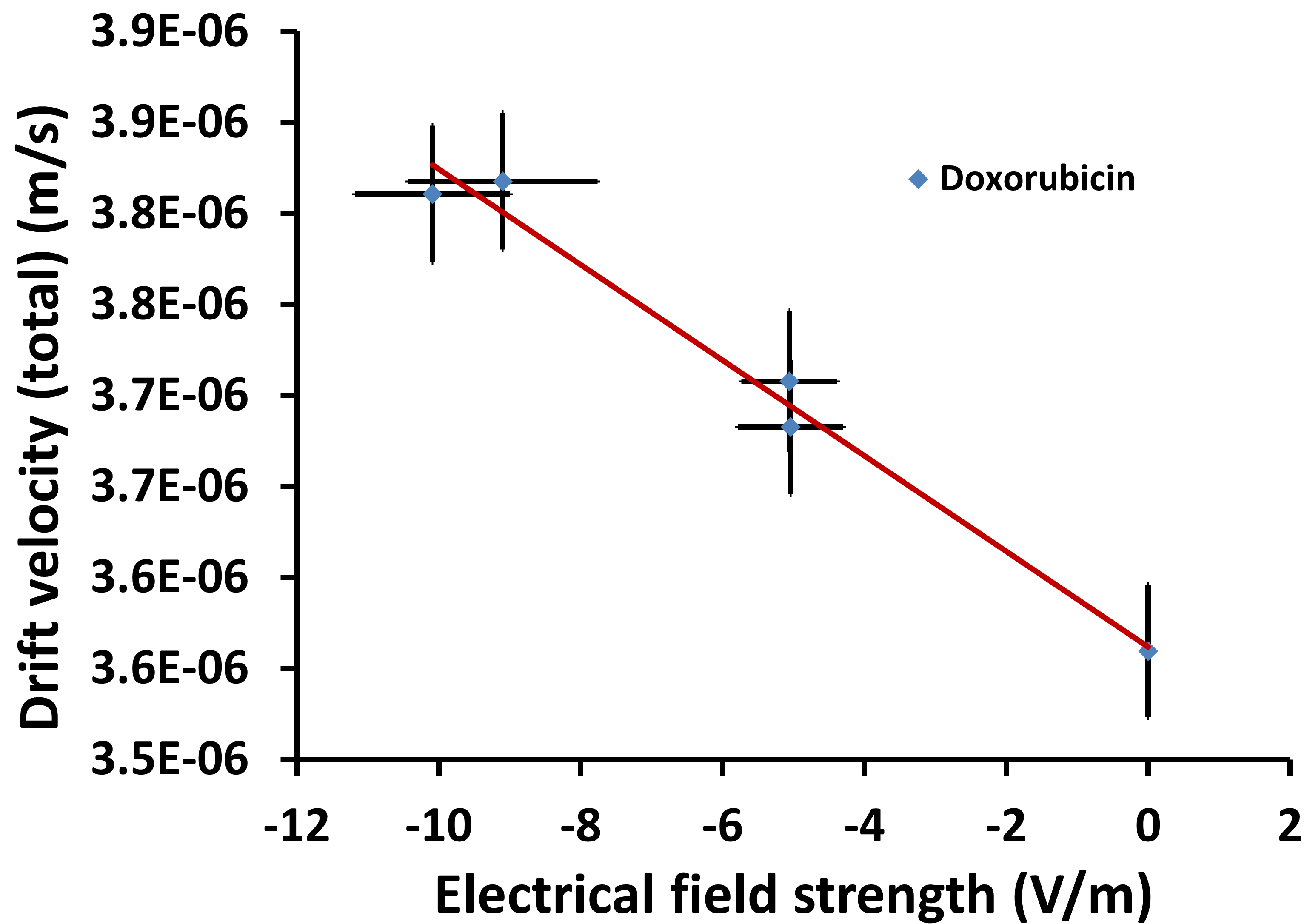
Plotting the calculated drift velocity against the applied electrical field strength (Figure 4) enables the determination of the electrophoretic mobility from which the surface Zeta potential can be derived using the Smoluchowski approximation. Obtained results indicate a negative surface Zeta potential of the Liposomal Doxorubicin sample in 0.5 mM NaCl (-34.3 mV ± 2.8 mV). The results from the EAF4-MALS experiment are summarized in Table 1.
Conclusion
EAF4-MALS is a powerful analytical technique for the characterization of nano-enabled pharmaceuticals such as liposomal drug formulations. Besides access to the size distribution, it also provides information about the surface charge even under physiological conditions. Enabling access to these crucial physico-chemical parameters, EAF4-MALS is an indispensable tool not only for quality control purposes but also when it comes to a more rational development of novel nano-enabled products in this extremely innovative field.
References
[1] Y. Barenholz, 2012, Journal of Controlled Release, 160, 117-134.
[2] J. Parot, F. Caputo, D. Mehn, V.A. Hackley, L. Calzolai, 2020, Journal of Controlled Release, 320, 495-510.
[3] ASTM WK68060 - New Test Method for Analysis of Liposomal Drug Formulations using Multidetector Asymmetrical-Flow Field-Flow Fractionation (AF4).