Christian Schwaferts, Reinhard Niessner, Martin Elsner, Natalia P. Ivleva(a)
(a)Institute of Hydrochemistry, Technical University of Munich
Introduction
The production and use of millions of tons of plastics worldwide have led to an uncontrolled release and accumulation of plastic debris all around our planet with yet unpredictable and multifaceted implications. Recent studies have revealed how plastic in the environment undergoes fragmentation even down to nanometric dimensions [1], however suitable methods for the analysis of such small plastic particle sizes are still scarce [2]. Here we present an innovative approach for the characterization of nanoplastic particles using Centrifugal Field-Flow Fractionation coupled with UV, Multi Angle Light Scattering detection and Raman Microscopy.
Hyphenation of Centrifugal Field-Flow Fractionation with Raman Microscopy
In Centrifugal Field-Flow Fractionation separation is induced by a centrifugal field that acts perpendicular to a rotating, ribbonlike channel. Hence, sample constituents are predominantly separated due to differences in their masses. Under laminar flow conditions, this means that less massive particles that diffuse faster against the applied centrifugal field, elute sooner than more massive particles (Figure 1).
Centrifugal Field-Flow Fractionation (CF3), just like any other Field-Flow Fractionation (FFF) technique, can be connected in-line with a multitude of powerful detectors. While UV-detection usually enables the quantification of a fractionated sample, Multi Angle Light Scattering detection provides information about its size distribution (CF3-UV-MALS); however, both detectors, unlike Raman Microscopy, are not able to provide insight into the chemical nature of a respective sample. Therefore, in order to add chemical identification capabilities to the CF3-UV-MALS setup, a dedicated Raman flow cell was developed enabling the hyphenation with Raman Microscopy (CF3-UV-MALS-Raman) [3].

Experimental Details and Results
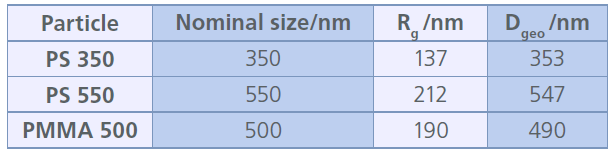
The performance of the CF3-UV-MALS-Raman setup was evaluated using a mixture of three different nanoplastic particles with varying sizes. This mixture included polystyrene (PS) particles of two different sizes, 350 nm and 550 nm (25 mg/L each; density: 1.05 g/mL), as well as poly(methyl methacrylate) (PMMA) particles of 500 nm (100 mg/L; density: 1.18 g/mL). Fractionation of the ternary nanoplastic particle mixture was achieved using an initial CF3 speed of 3000 rpm with exponential fi eld decay to zero over the course of 100 min.
Figure 2 displays the obtained CF3-UV-MALS fractogram. All three particle sizes were well-separated as indicated by the recorded UV-signal (left y-axis, blue line). In addition, the size of each individual particle fraction as radius of gyration (Rg) could be assessed by evaluating the respective MALS signals at each time interval of the fractogram using the sphere model fit (right yaxis, green dots). However, a distinct statement on the chemical identity of each particle fraction was impossible without evaluating the Raman signal.
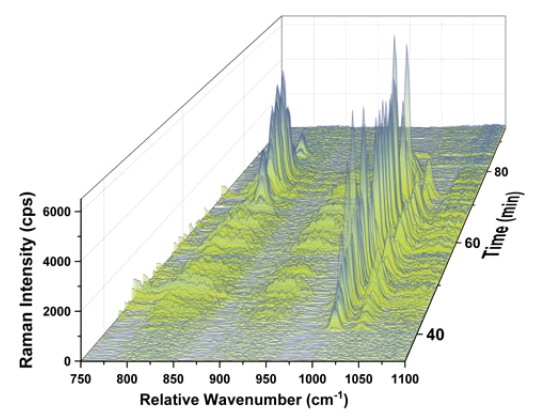
In order to get an idea of the chemical identity of each particle fraction, the Raman signal at each individual time interval was monitored using the characteristic Raman band of each polymer (PS: 1000 cm-1; PMMA: 812 cm-1). As highlighted in Figure 3, both PS particle fractions elute before the PMMA particles even though the nominal size of the PMMA particles is smaller (500 nm) than the size of the larger PS particles (550 nm). This nicely highlights that CF3 separates by mass and not by hydrodynamic size.
The obtained results are summarized in Table 1. In order to provide a better comparability of the calculated Rg with the nominal geometrical sizes provided by the manufacturers, obtained Rg values are converted into geometrical diameters (Dgeo) using the well-known relationship Dgeo = 2 x Rg / 0.775, which is valid for the nanoplastic particles investigated here, which can be considered as hard spheres.
Conclusion
The hyphenation of Centrifugal Field-Flow Fractionation with UV, MALS and Raman detection enables the simultaneous detection, sizing and chemical identification of polydisperse nanoplastic mixtures. Because of the wide range of polymers potentially present in nanoplastics, CF3 is an ideal separation technique as it can fractionate those particles with similar size but different mass or density. The design of the Raman flow cell used in this study takes advantage of the principle of optical particle trapping and can also be used in conjunction with other FFF techniques such as Asymmetrical Flow Field-Flow Fractionation. In addition, this setup is not restricted to the characterization of nanoplastic particles but can also be useful for nanomaterial characterization in general.
References
[1] H. Bouwmeester, P.C.H. Hollman, R.J.B. Peters, Environmental Science and Technology, 2015, 49, 8932−8947.
[2] C. Schwaferts, R. Niessner, M. Elsner, N.P. Ivleva, TrAC Trends in Analytical Chemistry, 2019, 112, 52-62.
[3] C. Schwaferts, V. Sogne, R. Welz, F. Meier, T. Klein, R. Niessner, M. Elsner, N.P. Ivleva, 2020, Analytical Chemistry, 92, 5813-5820.
Acknowledgements
Support by the SubμTrack project consortium as well as fi nancial support by the BMBF (BMBF Grant No. 02WPL1443F) are gratefully acknowledged.