Introduction
The U.S. National Institute of Standards and Technology (NIST) has produced a reference material monoclonal antibody (RM 8671 NIST mAb), intended for use in evaluating the performance of methods for determining physicochemical and biophysical attributes of mAbs [1]. It provides a representative test molecule for development of novel technology for therapeutic protein characterization. The NIST mAb is intended for a variety of uses that may include system suitability tests, establishing method or instrument performance and variability, comparing changing analytical test methods, and assisting in method qualification. In this work, the NIST mAb is used to compare separation, aggregation, quantification, and recovery parameters for Asymmetrical Flow Field-Flow Fractionation (AF4) versus Size Exclusion Chromatography (SEC), and to demonstrate the capabilities of the new Electrical Asymmetrical Flow Field-Flow Fractionation (EAF4) module (Figure 1) to simultaneously measure size and surface charge characteristics of antibodies and proteins.

Experimental Details and Results
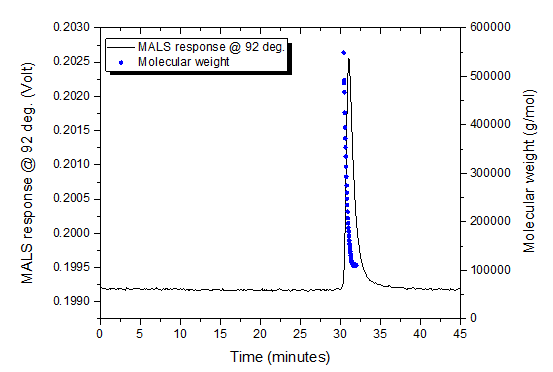
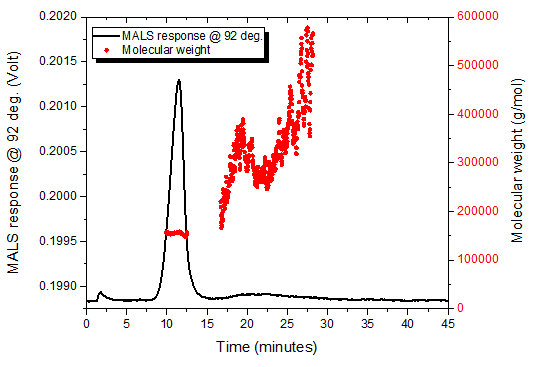
The NIST mAb was analyzed by SEC-UV-MALS (Figure 2) and AF4-UV-MALS to evaluate separation of monomer from aggregates and recovery by both techniques. The SEC columns used were Waters Ultrahydrogel 1000 and Ultrahydrogel 120 in series. Both analyses were performed using 1x phosphate buffered saline (PBS) as a mobile phase. Aggregates were not separated by SEC-MALS, but instead eluted with monomer in a single peak (Figure 2). Aggregates measured by AF4 (Figure 3) represented 10 % of the total mass injected as measured by UV-Vis (data not shown).
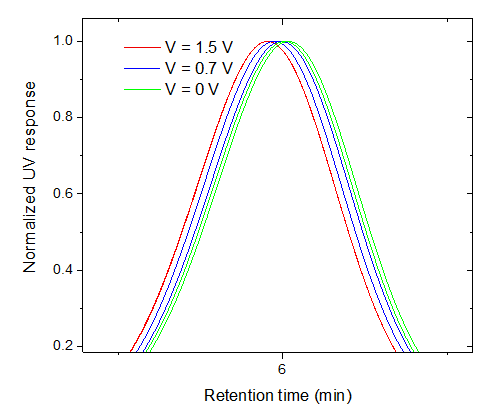
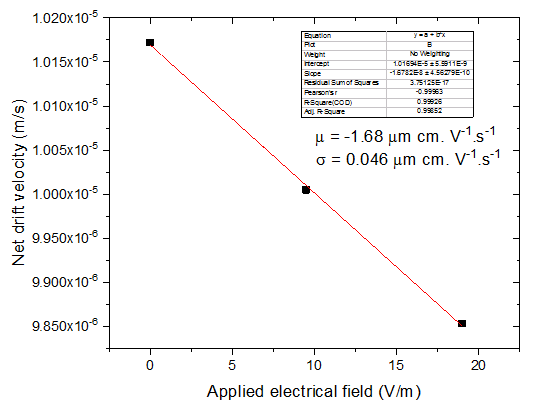
The NIST mAb was analyzed with EAF4-UV-MALS in 1x phosphate buffered saline (PBS) using a control run (no electric field) and two varied electric field strengths (Figure 4). The retention time shifted toward earlier elution with greater negative charge on the channel bottom, indicating the NIST mAb is negatively charged in 1x PBS. The shift in retention time can be related to the net drift velocity and plotted versus the applied electric field; the slope represents the electrophoretic mobility, which was measured to be -1.68 ± 0.05 μm cm V-1 s-1 under the experimental conditions in this study (Figure 5).
Conclusion
The Electrical Asymmetrical Flow Field-Flow Fractionation system allows both size and surface charge separation, enabling measurement of size or molecular weight distribution and electrophoretic mobility. The open channel design of FFF allows for better recovery of injected mass than SEC. This is important for quantifying protein/antibody aggregates present in small relative amounts, and represents an advantage of FFF over SEC for aggregate quantification.
References