Introduction
Alzheimer’s Disease is a degenerative brain disorder in which several biological dysfunctions are involved [1]. Among them, two main types of lesions have been discovered and widely studied: the neurofi brillary tangles and the amyloid plaques. These two lesions are caused by the aggregation and the accumulation of two proteins which are, respectively, the beta-amyloid peptide and the tau protein. The characterization of tau protein and its fi bril aggregates is important in the understanding of Alzheimer’s Disease, and is the focus of this study.
Experimental Details and Results
In this study, tau protein samples were analyzed by Asymmetrical Flow Field-Flow Fractionation (AF4) coupled with UV/Vis, Multi Angle Light Scattering (MALS) and Dynamic Light Scattering (DLS) detection to characterize 1) the protein monomer, 2) the protein fi bril aggregate, and 3) the protein fi bril aggregate after bath sonication. The aggregates were formed by adding heparin to the tau monomer to induce aggregation. The AF4 carrier solution was 20 mM MES, 100 mM NaCl, adjusted to pH 6.8 with NaOH.
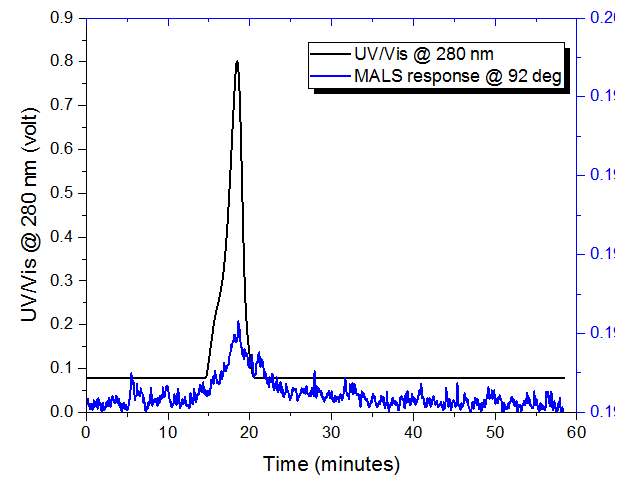
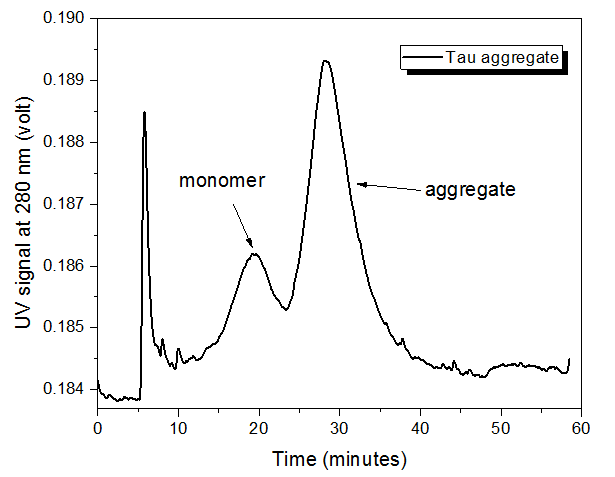
The tau protein monomer was analyzed with AF4-UV-MALS, but the concentration was too low to measure the molecular weight by MALS (Figure 1). The retention time of ~18 minutes for the monomer was also observed in the tau aggregate (Figure 2), indicating that monomer is still present in the aggregated sample.
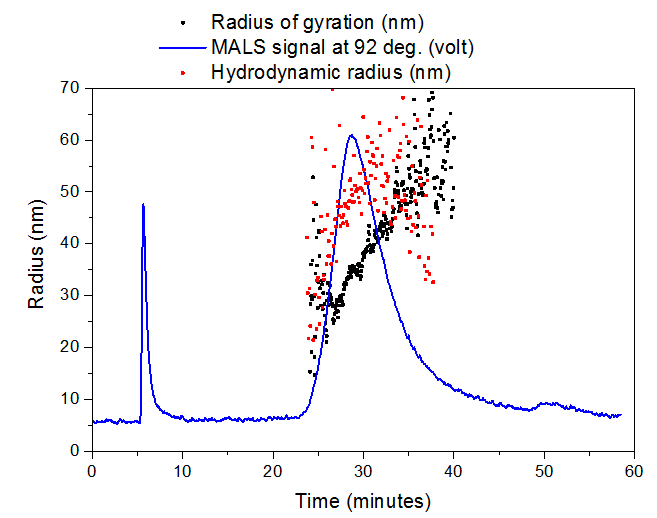
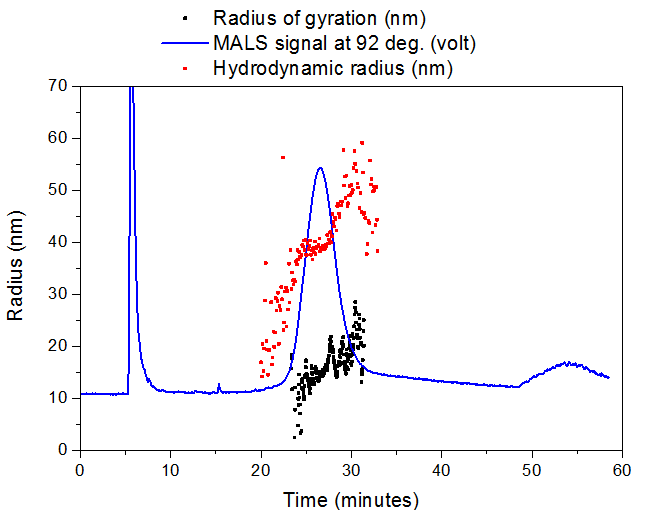
The aggregate sample had a mean radius of gyration (Rg) of ~35 nm and mean hydrodynamic radius (Rh) of ~50 nm, while the sonicated aggregate had a mean Rg of ~15 nm and mean Rh of ~40 nm. Sonication of the aggregate is likely shearing the long aggregate fi brils into shorter pieces with a smaller aspect ratio, resulting in a greatly reduced Rg but minimally reduced Rh. Slightly different elution times are observed for the sonicated (peak max at 26.6 min) vs. non-sonicated aggregates (peak max at 28.2 min), which agrees with the size change measured by Light Scattering.
Conclusion
Tau protein and its aggregate were characterized by AF4-UV-MALS-DLS. The tau aggregate had a bimodal distribution suggesting both monomer and aggregate were present in the sample. This demonstrates the ability of AF4 and Light Scattering to characterize tau protein monomers and their aggregates‘ size distributions, an important area in Alzheimer’s research.
References
[1] Jouanne M., Rault S., Voisin-Chiret A.-S., European Journal of Medicinal Chemistry, 2017, 139, 153-167.