Introduction
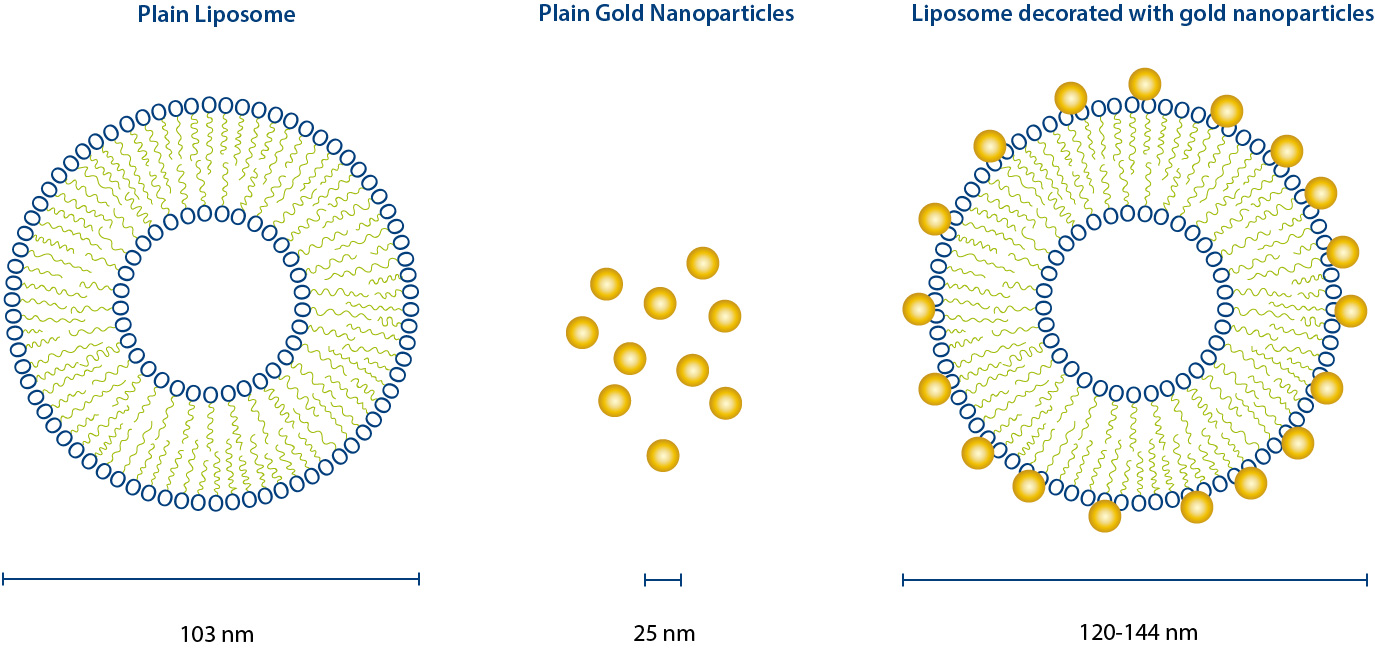
Nanoparticles are increasingly used for delivery of drug molecules, including liposomes and gold nanoparticles (AuNPs). Hybrid formulations containing both „soft“ (liposomes) and „hard“ (AuNPs) are of interest as well. Field-Flow Fractionation (FFF) is one of the most promising techniques, when it comes to the physico-chemical characterization of such potential drug delivery systems [1-4]. In this study, Centrifugal Field-Flow Fractionation (CF3) coupled to Diode Array Detector UV-Vis (DAD) was used to evaluate if AuNPs were associated with liposomes in solution (Figure 1). CF3-DAD was used for this study to separate constituents of the suspension by mass; the low-density liposomes and high-density AuNPs will have very different elution times, and thus CF3-DAD will be able to determine if the AuNPs are attached to the liposomes or freely suspended. As CF3 separates by mass, if multiple AuNPs are attached to a liposome their retention time will be greatly changed (toward later elution).
Principle of Centrifugal FFF
The channel is mounted on a centrifuge, which spins to generate a centrifugal field perpendicular to the channel flow. Less dense particles (e.g. liposomes) can diffuse back against the centrifugal field and elute sooner than denser particles, resulting in a mass separation.

Experimental Details and Results
Three samples were analyzed by CF3-DAD: a liposome-only sample (103 nm in size), AuNP-only sample (25 nm in size), and a mixture of liposomes and AuNPs. On the following fractogram graphs, the x-axis is time (minutes), the y-axis is wavelength (nm), and the signal intensity is a color heat map where purple is no signal and red is high signal. The liposome-only sample shows strong absorbance in the UV range (Figure 3), as expected for this sample. Since these particles are very low in mass, they elute at the beginning of the run (5 - 15 minutes) in what is known as a „void peak“ in FFF, similar to a solvent/system peak in chromatography. At the end of the run, a small amount of material, likely liposome aggregate, elutes as well.

The fractogram for the AuNP-only sample is shown in Figure 4. A small void peak at ~5 minutes is observed, absorbing in the 200 - 270 nm range. This is due to free citrate molecules that the AuNPs have as a surface coating to keep them stable in suspension. The main peak (15 - 40 minutes) has a strong UV response due to surface coating, and the AuNP signature at ~520 nm wavelength is due to surface plasmon resonance of the particles. Again some aggregate material elutes after the centrifugal field is removed (~57 minutes).

The fractogram in Figure 5 is from analysis of a mixture of liposomes and AuNPs. This sample was analyzed to determine if there is any attachment of multiple AuNPs onto the liposomes. The concentrations of each particle were lower than the individual samples, resulting in lower signal intensity. The liposomes elute early in the void peak, as seen in Figure 3. The AuNPs and their associated organic coating elute at ~25 minutes, as with the AuNP-only sample. This indicates that the Au NPs are not associating with the liposomes, and provides valuable information to drive future development of a „linker“ molecule to attach the AuNPs onto the liposomes.

Conclusion
Centrifugal FFF coupled to diode array UV/Vis detection was able to separate and characterize liposomes and Au-NPs by mass. The elution time for the AuNP signature surface plasmon resonance did not change in the mixture sample, demonstrating that they were not associated with liposomes. This is a promising application using centrifugal FFF for separation of drug-delivery particles, as the field of nanomedicine and nano drug delivery grows.
References
[1] M. Wagner, S. Holzschuh, A. Traeger, A. Fahr, U.S. Schubert, Analytical Chemistry, 2014, 86(11), 5201-5210.
[2] P. Iavicoli, P. Urban, A. Bella, M.G. Ryadnov, F. Rossi, L. Calzolai, Journal of Chromatography A, 2015, 1422, 260-269.
[3] S. Holzschuh, K. Kaeß, G.V. Bossa, C. Decker, A. Fahr, S. May, Journal of Liposome Research, 2018, 28(1), 22-34.
[4] L.O.F. Monteiro, A. Malachias, G. Pound-Lena, R. Magalhães-Paniago, V.C.F. Mosqueira, M.C. Oliveira, A.L.B. de Barros, E.A. Leite, Langmuir, 2018, 34(20), 5728-5737.