Introduction
Recombinant Antibodies are widely used in modern drug formulations. Asymmetrical Flow FFF (AF4) is the premier technique for the characterization of this kind of antibodies. The main reason for this is that AF4 can characterize not only the antibody species, but also their fragments and aggregates [1]. Furthermore, even small differences in the conformation and the general heterogeneity of samples can be determined.
Experimental Part
Fig. 1: Characterization of Antibodies with Aggregates and Fragments.
Fig. 1 reveals that using the Postnova AF2000 system not only the antibody species themselves, but also two fragments and higher aggregates could be separated and characterized. Moreover, the heterogeneity of the antibody’s fractions can be elucidated using this AF4 system as the peak shape is not symmetrical, but shows a broadening with higher retention times indicating a different conformation of the antibody species in this region.
Processing Temperature 30 °C
Fig. 2 shows the dependence of the antibodies from different processing temperatures. Thereby, the results clearly show that no fragments and aggregates can be detected when the antibody is processed at a temperature of 30 °C.
Processing Temperature 30 - 40 °C
However, at a slightly elevated processing temperature of 30 - 40 °C the antibody fraction already shows huge changes in its constitution. Two fragment peaks can be detected in the sample. This fragmentation process is caused by the increased temperature. The main antibody peak remains practically unchanged while a slightly increased peak broadening to higher elution times is observable. That shows that these antibody species with a different conformation are also increasing at a processing temperature of 30 - 40 °C. On top of that, a not negligible amount of aggregates is generated by the higher temperature indicated by the small peak eluting right after the main peak of the antibody.
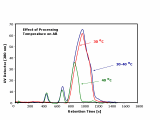
Fig. 2: Antibodies at different Temperatures
Processing Temperature 40 °C
Even more changes in the constitution of the antibody sample occur when the processing temperature is increased to 40 °C. Please note that the injection volume was smaller than in the measurements before. The main antibody peak changes dramatically as it signifi cantly decreases in size. Additionally, the antibody fraction with the different conformation is nearly completely decomposed. The fractogram shows an antibody peak, which is much more symmetric than the peaks discovered before. Furthermore, it is shifted towards earlier elution times indicating a now smaller size accompanied by a higher diffusion coefficient than before. Additionally, aggregates of the main antibody peak can be detected in this sample. However, it looks like that the pristine antibody is significantly degraded and has formed the increased amount of fragments, which can now be found in this sample. So, the increased processing temperature primarily causes a higher amount of fragments than an higher amount of aggregates.
Conclusion
In this study, we investigated the influence of temperature on the stability, fragmentation and aggregation of recombinant antibodies. Thereby, we could clearly demonstrate the excellent applicability of AF4 coupled with UV as a monitoring tool for temperature-induced antibody decomposition revealing that the antibody itself is rather decomposed to frag ments than to aggregates.
References
[1] Gabrielson J.P., Brader M.L., Pekar A.H., Mathis K.B., Winter G., Carpenter J.F., Randolph T.W., Journal of Pharmaceutical Sciences, 2007, 96(2), 268-279.