Introduction
Protein purification is an important step in biotechnology. There are a number of different purification techniques used in industry, where column chromatography, ultrafiltration and ultracentrifugation are mongst the most commonly used.
In this application note a new purification technique based on diffusion is demonstrated to purify a bovine γ-globulin solution with high throughput and efficiency. The theoretical basis of this approach was already established by Giddings and coworkers in the early 1990s [1,2].

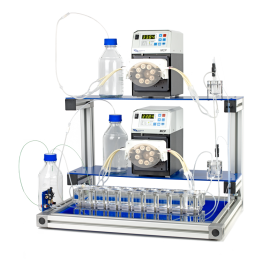
Figure 1 Top: A continuous SPLITT fractionation system with the purification cartridge and two peristaltic pumps. Bottom: A close-up picture of the purification cartridge.
The system setup (Figure 1) includes the purification cartridge and two peristaltic pumps for the delivery of the protein and buffer solutions. The purification cartridge consists of two inlet ports and two outlet ports. The inlet and outlet streams are divided by two physical splitters to ensure flow stability at the inlet and outlet sides. A sideview schematic of the cartridge is shown in Figure 2. The dashed lines represent the position of the inlet and outlet splitting planes (ISP and OSP) which are formed hydrodynamically in the cartridge as a result of the imbalanced flow rates at the inlets‘ and outlets‘ ports. The region between the ISP and OSP acts as a diffusion barrier (a virtual membrane) which separates the enriched protein from the impurity stream.

Figure 2: Side view schematic of the purification cartridge.
The sample solution is pumped continuously into the channel via the sample inlet port. The buffer solution is introduced from another inlet. The volumetric flow rate of the buffer solution is higher than that of the sample stream and that pushes the sample stream toward the top wall of the cartridge (Wall A). As the sample stream moves toward the outlet ports, a large fraction of the highly diffusive impurity can move through the diffusion barrier and exits the cartridge via the bottom outlet (impurity outlet). The protein component with a lower diffusion coefficient will mostly stay at the top region (between Wall A and ISP) and hence elute via outlet A. The dialysis-like process is quite fast (few tens of seconds) and the purified fraction can be continuously collected during the course of the purification.
Experimental
A 1:1 (w/w) mixture of bovine γ-globulin and 870 Da polystyrene sulfonate standard (used as a surrogate for low molecular weight impurity) was suspended in a 0.9 % (w/w) NaCl solution. The same NaCl solution was used as the buffer solution in the purification process. A 10 mL aliquot of the mixture was pumped into the cartridge at a flow rate of 1 mL/min. The purified fraction was passed through the cartridge two more times to reach the optimum purity of the protein solution. The aliquots collected from outlets A and B were analyzed by Asymmetrical Flow Field-Flow Fractionation (AF4) equipped with a UV detector.
Results and Discussion

Figure 3 Left: AF4-UV analysis of the original mixture and the fi nal purifi ed fraction. Right: Purity of γ-globulin in consecutive purification cycles.
Figure 3 left shows the results of the AF4-UV analysis of the original mixture and the final purified fraction. In the final purified fraction, the γ-globulin to impurity peak area ratio was increased by a factor of 5.2 when compared to that of the original mixture. The enhancement in the relative γ-globulin area is equivalent to a 97% purity in the final purified fraction (Figure 3 right).
Conclusion
The diffusion-based purification system is a fast, high throughput and cost-effective alternative to other protein purification methods. A single cartridge system can process up to 1.4 liters of protein solution (43 g protein) per day. The purification cartridge can be easily cleaned and is reusable indefinitely. The level of purity can be increased significantly by increasing the number of passes. The dilution per cycle is estimated to be 300%.
References
[1] Williams et.al, Ind., Eng., Chem., Res., 1992, 31, 2172-2181
[2] Levin et.al, Anal., Chem., 1993, 65, 2254-2261