Monoclonal Antibody Aggregates
Protein aggregation has become an important issue in the development and QC of pharmaceutical formulations and novel drugs. Drug activity, bioavailability and possible negative side effects such as adverse (auto) immune reactions can be directly related to the existence and quantity of aggregates in such biopharmaceuticals. Especially monoclonal antibodies are increasingly used as therapeutic agents. However, the formulation process involved may also lead to aggregated forms of the antibody, which could trigger possible negative immune responses [1].
Classical Characterization Techniques
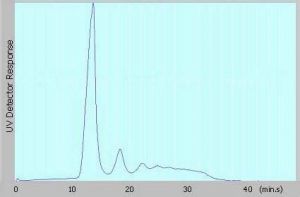
Figure 1: Fractogram of Antibody obtained from AF4-UV @ 280 nm
The analytical characterization of these antibodies remains a difficult and demanding challenge as monomeric, oligomeric and aggregated antibody species have to be separated at the same time. Established techniques such as Analytical Ultra Centrifugation (AUC) and Size Exclusion Chromatography (SEC) have been used for this purpose. However, these techniques also show some inherent limitations and potential problems when applied to antibody aggregate characterization.
AUC is able to provide a high resolution size separation, quantification and characterzation of antibody aggregates. However, the limited reproducibility, high capital and operation costs and the need for special highly-trained operators are quite limiting its popularity and the use of AUC as a standard analytical tool. Moreover, collection of the size-classed fractions for further analysis or activity determination is not possible.
Up to date, SEC is the most commonly used separation technique for characterization of antibody aggregates in biopharmaceuticals. The limitations of SEC are closely connected to the stationary phase, which is used inside the columns. Low recovery by adsorption of protein onto the column materials, shear effects changing the samples and damaging their bioactivity plus filtering of aggregates via the columns matrix can lead to inaccurate results at any time. Additionally, the organic modifier and eluents required by the special column packing can alter the antibodies and change the ratio of detected aggregates at the column outlet. Another limitation is the size exclusion limit of the column which restricts the upper molar mass and particle size range of the technique. As a consequence, there is a tendency to observe lower amounts of aggregates and smaller particle sizes in SEC than e.g., in AUC or FFF.
AF4 - The Modern Alternative
Asymmetrical Flow Field-Flow Fractionation (AF4) is an innovative separation technology, which clearly overcomes the problems associated with AUC and SEC and has become the modern alternative for all these characterization needs. AF4 is a separation technique using an unpacked separation channel instead of a packed column. Lack of a stationary phase provides unique advantages, which are: no shear forces, matrix interaction-free sample separation, fast separation times, and great flexibility with respect to the choice of solvent. Also, AF4 offers a wide separation range from 1 kDa / 1 nm up to several MDa / 10 μm. Thus, a complete picture of the aggregation phenomena is provided, including monomer, soluble, and insoluble aggregates and even the subvisible particle fraction.
Advanced AF4 Detection by MALS & DLS
As an elution-based technology, AF4 can be easily hyphenated with sophisticated on-line detectors for multi-dimensional information from a single injection. UV is typically used as a standard detector for aggregate identification and quantification. Additionally, Multi Angle Light Scattering (Postnova PN3621 MALS) and Dynamic Light Scattering (Malvern Zetasizer Nano DLS) are advanced detectors providing absolute molar mass and particle size as further characterization information about the separated aggregates. For these reasons, the FDA has recently recommended the use of AF4-MALS for any characterization of antibody formulations and may expect supporting data with any new application in the future [2].
Comparison of AF4, AUC and SEC Data
As an example for the performance of AF4 technology, data of the characterization of a monoclonal therapeutic antibody sample containing aggregates are shown in Fig. 1. The analysis was performed using a Postnova AF2000 Focus Asymmetrical Flow FFF with PN3211 UV Detection. In parallel to that the same sample also was characterized using AUC and SEC in order to highlight the differences. The obtained data for the amount of aggregates using the different methods are listed in Tab. 1 below.
| AF4 | AUC | SEC | |
| Amount Aggregates % | 28.00 | 24.00 | 1.00 |
Table 1: Amount of Aggregates obtained from AF4, AUC and SEC.
The results show that the amount of aggregates, which was determined with AF4 (28 %) and AUC (24 %) are quite similar and in good agreement with each other. However, the results obtained from SEC measurements clearly differ and only show an aggregate content of 1 %. This could indicate that the majority of the aggregates may have already been filtered out and degraded by the column material or even could have been adsorbed onto the column.
Unique Features of AF4
Although Asymmetrical Flow FFF offers similar information as SEC, the lack of packing material leads to significant advantages, especially low shear conditions preventing degradation of aggregates and no size exclusion limit. Versus AUC, the AF4 system provides much easier operation, is easily suited for autosampler use and allows the physical fractionation of separated sample material. In summary, the obtained results clearly show that AF4 is easier to handle in comparison to AUC and at the same time provides more accurate results for aggregates than SEC.
References
[1] Guidance for Industry on Immunogenicity Assessment for Therapeutic Protein Products Food and Drug Administration (FDA), April 2014.
[2] Draft Guidance for Industry on Immunogenicity-Related Considerations for the Approval of Low Molecular Weight Heparin for NDAs and ANDAs, Food and Drug Administration (FDA), August 2014.