Institute for Environmental Studies (IVM), VU University Amsterdam, The Netherlands
Introduction
Nanoparticles (NPs) are added to a large variety of daily-use personal care products to improve their properties and gain specific effects. For example, when used in toothpaste they are intended to support the cleaning process as well as to enhance the remineralization of teeth. Many types of NPs are found in personal care products depending on product type and manufacturer including NPs of hydroxyapatite, titanium dioxide, Na-Ca-Si-P bioglass or amorphous silica. Since there is only limited information about the nanoscaled content of the products, the consumer has to refer to and trust in common regulations. In this application note the characterization of a commercial biomimetic mouthwash is demonstrated.
Nanoparticles in Mouth Washes
Commercial mouthwash formulations are often quite complex, consisting of water, alcohol, dyes, flavourings as well as potentially containing NPs. Biomimetic approaches have been used to develop NPs for inclusion in a variety of oral healthcare products including nanoapatites for biofilm management at the tooth surface and NPs for the remineralization of early enamel lesions. In the mouthwash used in this study, Zn-hydroxyapatite and amorphous silica NPs are intended to enhance the remineralization process and promote the occlusion of submicron dental fissures.
The Experiment
To investigate the abundance of NPs in the mouthwash, before and after use, by analysis of the solutions using asymmetric flow field-flow fractionation (AF4) coupled to both dynamic light scattering (DLS) and ultraviolet (UV) detectors.
Four independent samples were analyzed:
1) The original mouthwash solution
2) The mouthwash after 1 min of rinsing in the mouth
3) The mouthwash after 3 min of rinsing in the mouth
4) Blank saliva sample before mouthwash use
Mouth Wash - Use, Extraction and AF4-UV-DLS Analysis
For sample preparation, 5 mL of the original mouthwash was diluted 1:10 (v/v) with 0.2 % NovaChem (v/v) and filtered through glass wool. The saliva was prepared in a similar way. For the time dependent measurements, 5 mL of mouth wash was used, rinsed in the mouth for one and three minutes, collected, the volume recorded, diluted and filtered as above. All samples were subjected to AF4 analysis using an AF2000 equipped with UV and DLS detectors. Online detection using DLS was facilitated by direct coupling of the DLS into the AF4 detector flow stream with a special quartz flow cell. A 0.2 % NovaChem (aq.) solution similar to the sample conditions was chosen as carrier liquid and a constant detector flow rate of 0.5 mL/min was set.
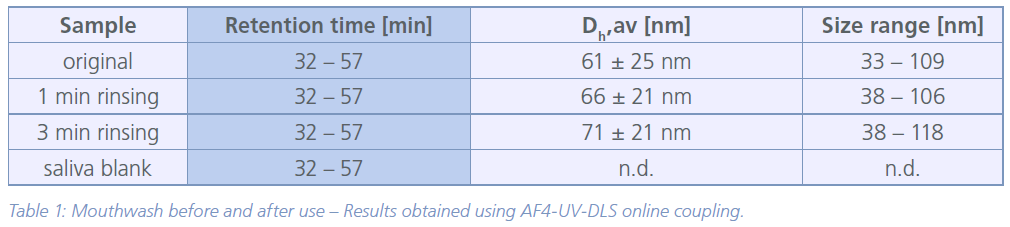
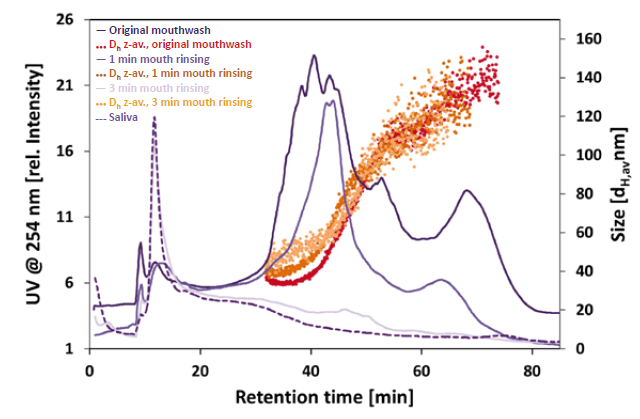
DLS coupled to a UV detector proved to be suitable to detect and measure the size of the particles and account for proteinaceous components of the saliva containing samples. The fractograms (Figure 1) clearly show the NPs of the mouthwash before and after use. The original mouthwash revealed NPs in the size range of 33 – 109 nm (Z-average hydrodynamic diameter, Dh z-av.). Although, the average particle diameter was comparable after 1 min of mouth rinsing (retention time 32 – 57 min), less small primary NPs were detected (retention time 32 – 48 min). Also, a slight increase in particle size of approximately 10 nm in diameter after rinsing could be observed. Based on the recorded UV signal intensity, about 40 % of the particles were detected compared to the original mouthwash after 1 min of rinsing and only 3 % after 3 min of rinsing. Hence, it can be assumed that the NPs are either lost due to strong agglomeration with saliva (proteins) and subsequent filtration (glass-wool) or they remain in the mouth region. No NPs were detected in the pure saliva sample. The measured particle sizes and detailed information are given in table 1.
Conclusion
AF4 coupled to UV and DLS proved to be suitable to analyze the NP content of a commercial biomimetic mouthwash. By comparison of the UV fractograms, we can see a 60 % decrease in the concentration of particles after 1 min of mouth rinsing, whereas 97 % of the particles were “lost” after 3 min. The DLS data shows some small changes in particle size and size distribution of the particles after rinsing in the mouth which may be due to agglomeration in the saliva. NPs were not found in the pure saliva sample but it clearly shows the proteinaceous compounds eluting in the early part of the separation (10 – 35 min). The fate of the NPs was not targeted and may be the subject of forthcoming investigations. For further studies the user may utilise the hyphenation of AF4 with ICP-MS to identify NP composition and distinguish between inorganic and organic matter in complex samples or matrices.
Reference
[1] Peetsch, A., Epple M., Characterization of the solid components of three desensitizing toothpastes and a mouth wash. Mat.-wiss. u.Werkstofftech. 2011, 42, 131-135.
[2] Hannig M., Hannig C., Nanomaterials in preventive dentistry, Nature Nanotechnology, 2010, 5, 565-569.