Postnova Analytics has published a new poster that compares the suitability of Electrical Asymmetrical Flow Field-Flow Fractionation (EAF4) and Size Exclusion Chromatography (SEC) for determining the physicochemical and biophysical attributes of monoclonal antibodies.
In the described work, a reference monoclonal antibody (RM 8671 mAb), from the U.S. National Institute of Standards and Technology (NIST), was used to compare separation, aggregation quantification, and recovery parameters for an EAF4-UV-MALS versus SEC-UV-MALS techniques. The NIST mAb provides a representative test molecule for development of novel technology for therapeutic protein characterization.
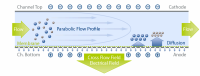
The poster describes how the EAF4 module enabled simultaneous measurement of size and surface charge characteristics (electrophoretic mobility) of antibodies and proteins. Further while no aggregates were detected by SEC, the FFF system showed protein / antibody aggregates represented 10% of the total mass injected. The researchers concluded that the FFF open channel design may allow for better recovery of injected mass than SEC which is particularly important when seeking to quantify aggregates present in small amounts.
Postnova Analytics EAF4 technology uniquely combines the principle of Electrical and Asymmetrical Flow FFF in just one system. In an EAF2000 system - Electrical and Cross Flow Fields are applied simultaneously across the FFF channel enabling separations by size and particle charge based on electrophoretic mobility. Combining these two powerful separation techniques in a single platform opens the door to characterising complex proteins, antibodies and viruses as well as environmental and charged nanoparticles or polymers that have proven intractable to other techniques.